Unusual trinuclear complex of copper(II) containing a 4′-(3-methyl-2-thienyl)-4,2′:6′,4″-terpyridine ligand. Structural, spectroscopic, electrochemical and magnetic properties
Résumé
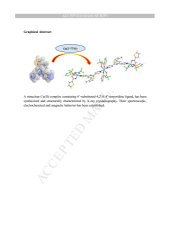
We report the synthesis, characterization, crystal and molecular structure as well as the spectroscopic, electrochemical and magnetic properties of an unexpected trinuclear copper(II) complex (1), made of three Cu(2-TTA)2 units (2-TTA = 2-thenoyltrifluoroacetone) bridged by two 4'-(3-methyl-2-thienyl)-4,2′:6′,4″-terpyridine (4-stpy) ligands. The central Cu(II) atom shows an octahedral geometry, while the lateral metal centers present a slightly distorted square pyramidal coordination sphere. It is suggested that the introduction of the relatively bulky substituent groups (2-thienyl and [sbnd]CF3) in the 2-TTA ligand are responsible of this uncommon coordination mode. The magnetic behavior of 1 is reported in terms of a combination of monomer and dimer units, leading to weak antiferromagnetic interaction (J = −1.93 cm−1). The cyclovoltammogram of 1 exhibits, in the cathodic potential region, three redox events due to Cu2+/Cu+ and Cu+/Cu0 redox couples and to the mono-electronic reduction of the 4-stpy ligand.
Fichier principal
 Toledo et al. - Unusual trinuclear complex of copper(II) containin.pdf (4.31 Mo)
Télécharger le fichier
mmc1.docx (2.03 Mo)
Télécharger le fichier
mmc1.pdf (462.81 Ko)
Télécharger le fichier
Toledo et al. - Unusual trinuclear complex of copper(II) containin.pdf (4.31 Mo)
Télécharger le fichier
mmc1.docx (2.03 Mo)
Télécharger le fichier
mmc1.pdf (462.81 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)

